MAKE A MEME
View Large Image
| View Original: | Tetrahedral Structure of Water.png (500x448) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
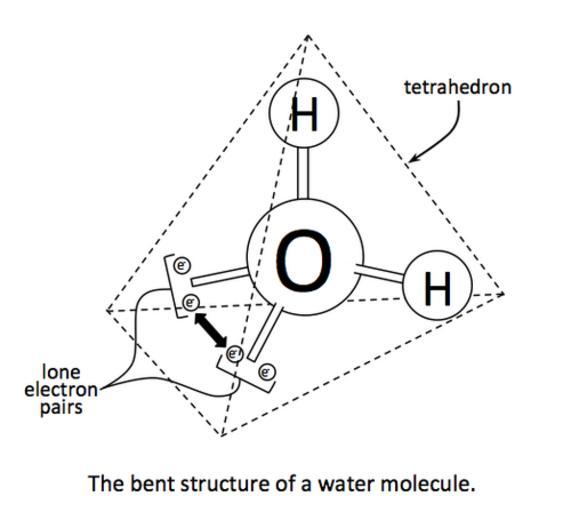
| Keywords: Tetrahedral Structure of Water.png en Water H2O is actually a bent molecule Oxygen has two lone pairs of electrons that do not bond with another atom Since water is a tetrahedral molecule the lone pairs must be adjacent to each other When this happens the lone pairs exert a repulsive force on each other effectively bending the entire molecule This bent nature generates polarity in the water molecule 2014-04-24 12 39 00 own Thebiologyprimer cc-zero Uploaded with UploadWizard Water molecule Hydrogen bonding Electronegativity | ||||