MAKE A MEME
View Large Image
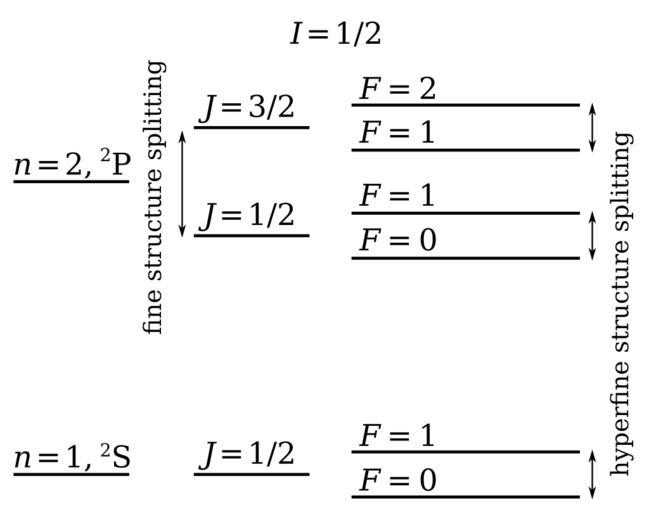
| View Original: | Fine hyperfine levels.svg (432x341) | |||
| Download: | Original | Medium | Small | Thumb |
| Courtesy of: | commons.wikimedia.org | More Like This | ||
| Keywords: Fine hyperfine levels.svg Fine and hyperfine structure in hydrogen The coupling of the different angular momenta leads to energy level splitting Not drawn to scale The electron spin angular momentum S is coupled to the electron orbital angular momentum L to form the total electronic angular momentum J This is subsequently coupled to the nuclear spin angular momentum I to form the total angular momentum F The term symbol takes the form <sup>2S+1</sup>L with the values of L represented by letters S P D F G H 0 1 2 3 4 5 so that for instance a <sup>2</sup>P term represents a state with S 1/2 and L 1 The single electron in a 1s subshell gives rise to the <sup>2</sup>S term L 0 and S 1/2 can only combine to give J 1/2 This in turn can combine with the nuclear spin I 1/2 to give total angular momentum F 0 1 The single electron in a 2p subshell gives rise to the <sup>2</sup>P term L 1 and S 1/2 can combine to give J 1/2 and J 3/2 These can combine with the nuclear spin I 1/2 to give total angular momenta F 0 1 and F 1 2 respectively The hyperfine splitting of the ground <sup>2</sup>S state is the source of the 21 cm hydrogen line important in astronomy File Fine_hyperfine_levels png originally created by User DJIndica Converted to SVG by w User Edudobay w User Edudobay 2010-05-16 Radio astronomy History of physics Cosmology Spectroscopy Hydrogen physics | ||||